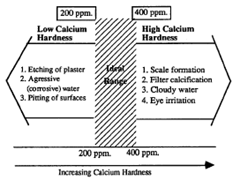
Total hardness is the measure of calcium (Ca) and magnesium (Mg) in the water. Excessive hardness—the combination of calcium [Ca] and magnesium [Mg]—causes calcium scale to build up on the walls and floor of plaster finished pools and spas. It also leaves scale build-up in heaters, heat exchangers, and other filtration components. Recognize that it is not the magnesium that forms the scale, only the calcium forms scale. Therefore in the pool industry, the focus is on maintenance of calcium levels.
Generally, low calcium hardness presents a larger problem to pools than high calcium hardness does. When the hardness level drops too low, the water becomes aggressive and will cause corrosion, pitting of plaster, and grout to dissolve. If pH, calcium hardness and total alkalinity are low, the corrosiveness and aggressiveness of the pool water will be greatly increased.
Control of scaling or aggressive water requires the calcium hardness level to be kept above 200 ppm and below 400 ppm. The suggested range is 200-300 ppm. Use the Langelier saturation index calculation to determine if the pool water is either aggressive (low hardness level) or scale forming (high hardness level).
Pharmaceutical grade calcium chloride (CaCl) is used to increase the hardness level. Because calcium chloride produces a significant amount of heat, the total amount needed should be divided into half and applied to the pool in two separate but equal doses. Remember when dissolving chemicals, add chemicals to water; never add water to chemicals.
To reduce the calcium levels, dilution is recommended. Remove some of the pool water and replace it with fresh. Trucked-in water from an alternate source may be the answer to control hardness. Calcium can make up as much as 75% of the total hardness with the remainder being primarily magnesium.
To test for hardness, a water sample is taken from at least 12 inches below the surface of the water. This volume of sample water is treated with a calcium buffer and then a dye. A reagent is then added to the sample one drop at a time and mixed. The number of drops it takes to produce a complete color change, from red to blue, is then multiplied by a constant provided by the test kit manufacturer. This gives the pool operator the ppm concentration of calcium. In conducting hardness tests, proceed slowly and allow enough time to mix the sample after each drop is added.
Testing for hardness may have special problems. It is possible that the color change will never take place. This indicates the presence of interference, probably copper. To remedy this, add a few drops of hardness reagent before adding the buffer and indicator. The number of drops added should be included in the total number of hardness reagent drops to obtain the complete color change.