Alkalinity in water represents the amount of bi-carbonates, carbonates, hydroxide and sometimes borates, silicates and phosphates. Total alkalinity is the resistance of water to changes in pH. The higher the total alkalinity, the more difficult it is to change the pH with soda ash or acid.
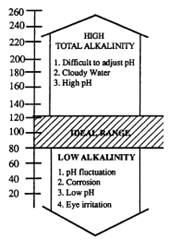
Testing for total alkalinity is essential to make proper determinations of the saturation index (see chapter 6) as well as for bather comfort and ease of pH control. Total alkalinity (calcium carbonate) should be kept between 80-120 ppm for pools with inert liners, and between 100 to 125 ppm for pools with plaster finished surfaces.
The pool water total alkalinity is measured using a test kit. Although test kits vary, the procedure is basically the same. A quantity of water is placed in the viewing cell and alkalinity indicator is added. This indicator produces either a blue or green color when calcium carbonate (alkalinity) is present. A reagent is then added to this mixture using a dropper or measuring device. The operator must count the number of drops necessary to change the color of the test sample from blue or green to a reddish, amber color. The color change represents the neutralizing of the alkalinity.
Total alkalinity (ppm.)
Determine alkalinity by multiplying the number of drops from the dropper by 10. Each drop represents 10 ppm per drop. For example 10 ppm x 11 drops would equal a total alkalinity of 110 ppm.
Testing for total alkalinity has special problems. When using a reagent which is older than its one-year shelf life, the test indicator may give different or opposite color readings. Therefore, replace the reagent annually and discard the old reagents.
It is recommended that the results of total alkalinity be considered before adjusting pH. It is essential to maintain a total alkalinity of 80-120 ppm in swimming pools to maintain a stable pH. The direction of pH change, or even the need for adding chemicals is greatly influenced by the level of total alkalinity. Total alkalinity does not vary quickly.